Okay, so now we see that this is day-to-day temperature data for just a refrigerator. There aren't any models specified – the form has an ID that should ideally website link back into the SOP associated with it, so Potentially we’ll have the units being recorded from that. There’s no products ID, yet again with any luck , this is the only refrigerator in the laboratory.
Data needs to be arranged chronologically with apparent time and day stamps for almost any additions to the original report.
By following these methods, pharmaceutical companies can safeguard their data towards breaches in integrity.
These principles collectively make sure the integrity, dependability, and traceability of data, making them fundamental in fields that demand from customers significant benchmarks of documentation and data administration.
Reliable: Making certain a seamless, chronological sequence of recorded situations with data and time stamps for trustworthy data audit trails.
How a system handles the legibility of and adjustments to raw data is vital, and will be regarded as through the early style and design analysis and validation phases of any new method. User requirements, click here technical specs and testing really should incorporate assessments for raw/source data immutability, data change Command and audit trails.
一貫性とは、全記録に矛盾がないこと。データのライフサイクルを通じて、どのプロセスにおいても欠損や不整合、改竄などが生じないようにするための要件。
Copies of electronic media might be established comparatively easily and on a significant scale. With out thorough Corporation, several scenarios may perhaps result in issues as to that's the correct, unique document.
Likewise, documents must be capable to be linked back again on the products applied to make them, like their validation point out, upkeep and calibration documents, and any configurations that were Energetic throughout the recording.
Which must be recorded for a specific software will depend on what you’re recording. entry matching enough time on the observation. The more aid for contemporaneous recordings the technique provides, the higher.
You might measure software program excellent in terms of examination protection or defects for every line of code. For GLP research and GCP trials, the solution is the final report and we measure its excellent concerning the data supporting the report’s conclusions.
FDA along with more info other regulators see a similar difficulties pop up time and time all over again. Numerous of those examples ended up taken from publicly out there FDA warning letters, but there's tiny doubt that EU regulators see precisely the same troubles. They generally fall into four categories.
(GCP) are variously defined by the regulations and steerage paperwork. For our reasons They're equal and suggest the first file of the initial observation
Data integrity is critical to all validation procedures in pharmaceutical and professional medical product producing services.
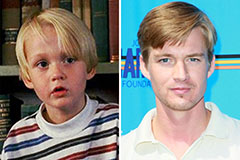 Mason Gamble Then & Now!
Mason Gamble Then & Now! Yasmine Bleeth Then & Now!
Yasmine Bleeth Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now! Heather Locklear Then & Now!
Heather Locklear Then & Now! Richard Dean Anderson Then & Now!
Richard Dean Anderson Then & Now!